Hydrogen-Deuterium Exchange Mass Spectrometry
A scenario for Protein A with two ligand binding sites
Written by NIKHIL KUMAR TULSIAN OF THE PROTEIN AND PROTEOMICS CENTRE, DEPARTMENT OF BIOLOGICAL SCIENCES, NATIONAL UNIVERSITY OF SINGAPORE
Small molecules or ligands bind to proteins at specific binding sites (orthosteric sites), and may lead to conformational changes at an alternative/distal (allosteric) site. Characterization of the protein-ligand interactions is essential to understand the effects on structural and conformational dynamics of the protein(s). Hydrogen-deuterium exchange mass spectrometry (HDXMS) is a solution-based biophysical method that offers insights into effects of physical (temperature, pH) and chemical (proteins, ligands, small molecules, lipids, and osmolytes) perturbations on protein(s) conformation.
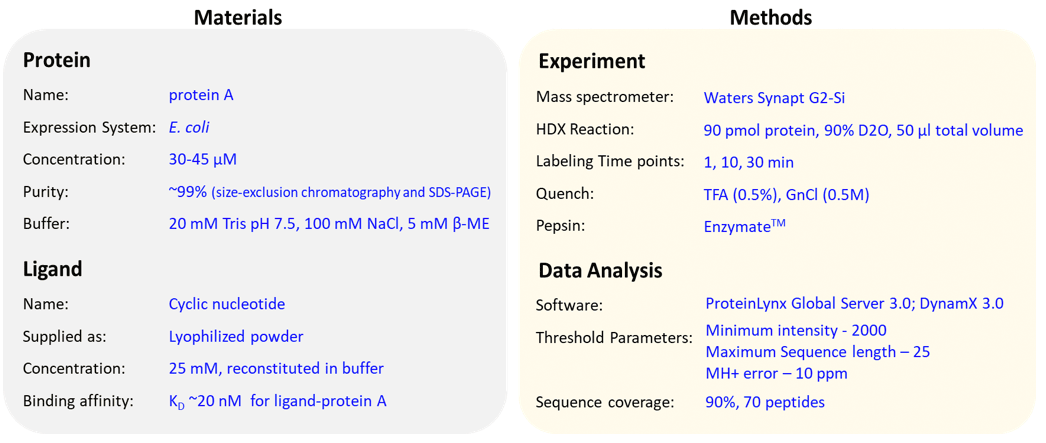
In this example, our objective is to monitor the conformational dynamics of protein A which consists of two high-affinity binding sites. To determine the effects of ligand-binding, a HDXMS experiment of protein A is performed in the presence and absence of ligand. Prior information of affinity of ligand binding to the protein A is useful to optimize their stoichiometric ratios. Here, the ligand binds to protein A with high affinities (KD ~nM) at both binding sites. High-resolution structures of protein A bound to the ligand has been solved, and is useful to ‘map’ the deuterium exchange kinetics on protein A. In this scenario, HDXMS results for ligand-bound protein A (condition B) showed decreased deuterium exchange at both binding sites suggesting that these regions undergo conformational rigidity and were protected against hydrogen-deuterium exchange, compared to protein A (condition A). Importantly, the HDXMS results also identified a distal region, which showed lower rates of deuterium exchange indicating that the binding of ligand induced changes in the conformational dynamics in other regions of the protein.
Materials
Materials supplied by you:
- Protein: A minimum of 100 pmol or protein per hydrogen-deuterium exchange reaction. Protein must have ~99% purity, and obtained after size-exclusion chromatography.
- Ligand: Concentration of ligand should be atleast 50 times more than the binding affinity.
- Buffer: Aqueous buffer (~50 ml). Maximum concentration of DMSO (5%), glycerol (5%), detergents (0.1%), lipids (25 mM).
Materials supplied by SingMass:
- Deuterated buffer: Aliquots (~200 µl) of aqueous buffer is subjected to speed vacuum drying to remove water and obtain ‘dry buffer’. This will be reconstituted in 200 µl of 99.99% deuterium oxide (D2O) to obtain ‘deuterated buffer’.
- Quench: A ‘quench’ buffer is required to lower the pH of the reaction to 2.5 and minimize deuterium-hydrogen exchange. The quench is composed of Trifluoroacetic acid (TFA) and water. For proteins with disulphide bonds reducing agents such as Dithiothreitol (DTT) or Tris (2-carboxyethyl) phosphine (TCEP) is used. For antibodies and viruses, 1.5 M Guanidinium Hydrochloride is used to unfold the proteins.
- Immobilized Pepsin column, Solvents (Water, Acetonitrile, Methanol).
Experimental Proceedure
Typical HDXMS experiment
A typical hydrogen-deuterium exchange mass spectrometry (HDXMS) experiment involves the following steps (Figure 1):
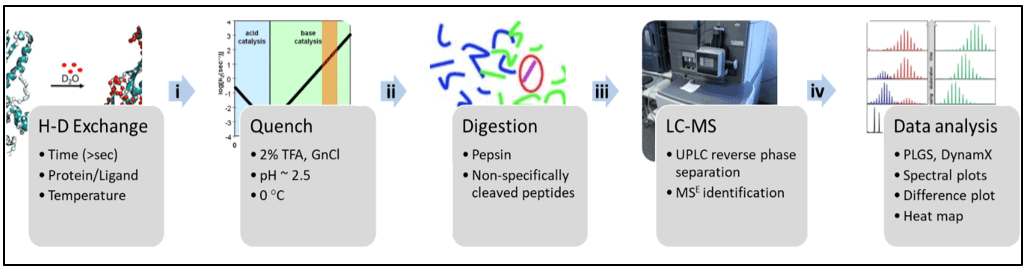
Figure 1: Workflow of hydrogen-deuterium exchange mass spectrometry experiment.
Step 1: Hydrogen-deuterium exchange reaction
Protein in the absence and presence of ligand is incubated in deuterated buffer for different labeling time points (e.g. 1, 10, 30 min).
Step 2: Quench and pepsin digestion
Hydrogen-deuterium exchange reaction is minimized by lowering the pH to ~2.5 and temperature to ~0 °C using a pre-chilled ‘quench’ solution. This quench solution consists of TFA (0.5%) and GnCl (0.5 M) to lower pH and unfold protein for pepsin cleavage. Acid-stable proteases like pepsin is used to non-specifically cleave protein into smaller fragments and obtain overlapping peptides.
Step 3: Chromatographic separation and Mass Spectrometry
Various peptides are subjected to reverse-phase liquid chromatography and these separated peptides are injected into mass spectrometer to perform LC-MS analysis. All peptic peptides within a specified mass range are detected and identified by MSE method in data-independent manner. LC coupled to MS is performed on Waters Synapt G2-Si mass spectrometer.
Step 4: Data Analysis
- Optimization of digestion and peptide identification:
After the mass spectrometric analysis, the peptides are identified using a software that searches for pepsin-digested peptides against the primary amino acid sequence databank. The undeuterated control samples of protein are used for peptide identification and mass assignment. - Deuterium exchange analysis of the samples:
All mass spectrometry ‘.raw’ files for deuterated and undeuterated reactions are analyzed using DynamX 3.0 software (Waters Corporation). The amount of deuterons exchanged by a peptide in deuterated samples are compared to the undeuterated samples. The deuterium uptake values are an average of three independent measurements. - Data output and representation:
The deuterium exchange kinetics of various peptides analyzed are then reported as deuterium uptake plots (Fig. 2A) and mass spectral envelopes (Fig. 2B). Different states of the protein, i.e., with and without ligand can be plotted as a ‘difference plot’ (Fig. 3A) and can be mapped onto structure of the protein to obtain a ‘heat map’ (Fig. 3B).
Current scenario
Results
In this study, we have mapped the effects of binding of ligand ‘cyclic nucleotide’ to protein A using HDXMS. Optimized experimental conditions yielded 70 peptides in the final analysis, spanning ~90% of the primary sequence of the protein. Firstly, we monitored the intrinsic dynamics of protein A in the absence of ligand and observed that most regions of the protein showed a relative fractional deuterium uptake of ~45%, indicating it to be conformationally dynamic protein. Next, we compared the deuterium exchange kinetics of protein A in the presence and absence of ligand, and is shown as a ‘difference plot’ in Fig. 2A which plots the differences in absolute deuterons exchanged in ligand-bound protein (condition B) and ligand-free protein (condition A). As shown, different regions behave differently, with certain regions showing increased deuterium uptake (red box, Fig. 2A) in ligand-bound protein A, no significant differences between the two conditions (yellow box), and decreased deuterium exchange (blue box, Fig. 2A) in ligand-bound protein. Differences in deuterium exchange observed at 10 min of labeling time were mapped onto high resolution crystal structure of ligand-bound protein A (Fig. 2B). This ‘heat map’ enabled a global visualization of the effect of ligand binding on the tertiary structure of protein. HDXMS difference map highlighted that the peptides showing decreased deuterium exchange are localized near the binding sites of the ligand.
Importantly, the heat map also revealed that a peptide (spanning residues 200-212) showed significant reduction in deuterium uptake. This peptide spanned regions distal to the ligand binding site, and decreased deuterium exchange across this region suggested an allosteric relay. Earlier studies have reported that this helical region is essential for binding of ligand to protein A. HDXMS results reveal that this region helps protein A to undergo a conformational change from a ligand-free to a ligand-bound form. Deuterium uptake of this peptide showed large differences across all deuterium labeling time scales (Fig. 3A). The isotopic distribution of the mass spectral envelopes of this peptide for the two protein conditions is also represented (Fig. 3B). These HDXMS results reveal that ligand binding induced conformational rigidity across this region, and hence decrease in deuterium uptake.
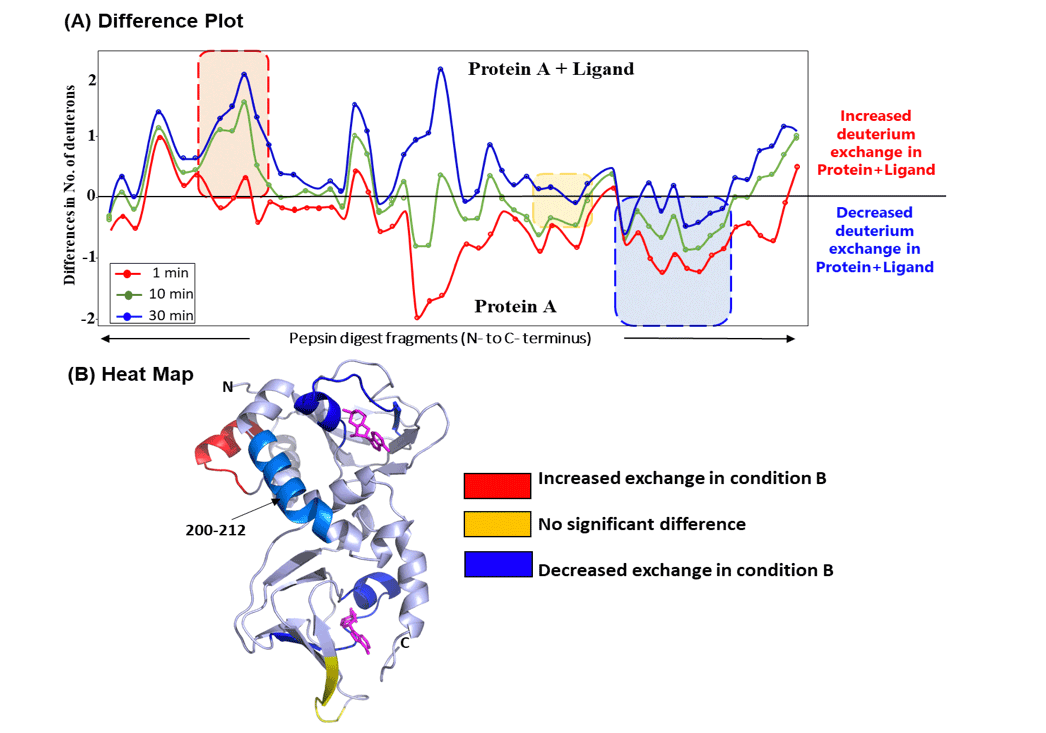
Figure 2: HDXMS reaveals global changes in ligand-bound protein A.
(A) Plot of differences in number of deuterons exchanged between ligand-bound versus free protein A. The difference plot displays the differences in deuterium exchange (Da) protein-wide where each dot represents a pepsin fragment peptide, listed from the N- to C-terminus (X-axis). Different deuterium labeling times 1, 10 and 30 min are colored according to the key. Positive differences represent increased exchange (highlighted by red box), and negative differences indicate decreased deuterium exchange (blue box) in ligand-bound protein, compared to ligand-free protein A. Certain regions with no significant differences are highlighted in yellow. (B) Deuterium exchange differences at 10 min labeling time were mapped onto the high resolution crystal structure of protein A. This ‘heat map’ highlights regions showing increased and decreased exchange, as indicated in key. Peptide spanning residues 200-212, distal to the ligand binding sites is highlighted in light blue. Ligands (magenta) are shown in sticks.
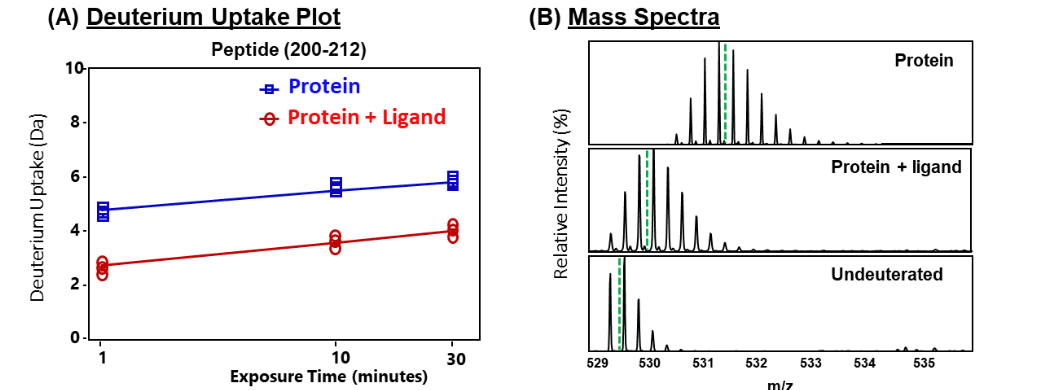
Figure 3: Deuterium exchange kinetics of peptide spanning residues 200-212.
(A) Deuterium uptake plot depicting the amount of deuterium (daltons, Da) uptake in free (blue) and ligand-bound (red) protein A at various labeling time points (1, 10, 30 min) for a peptide spanning residues 200-212. Data represents an average of three deuterium exchange experiments, with error bars too small to be visible. (B) Stacked mass spectra of the peptide (200-212) at 10 min deuterium labeling time highlighting the shifts in the isotopic distribution envelope of free and ligand-bound protein A, with undeuterated as reference. Green dashed lines indicate the centroid values. Deuterium uptake of the deuterated peptides is the difference between the centroid value of the deuterated sample and the centroid of the undeuterated sample.
Summary
Hydrogen-deuterium exchange mass spectrometry (HDXMS) results reveal differences in conformational dynamics of protein A in the presence and absence of ligand. Region 200-212 is shown to be essential for a conformational switch from a ligand-free and ligand-bound form.
